NEW DELHI: As India works towards becoming a $500-billion pharmaceutical powerhouse by 2047, scientists and industry captains are calling for a shift towards cruelty-free, science-driven drug testing methods that can speed up innovation and improve patient safety.A new report, ‘Landscape Analysis on Alternatives to Animal Testing for Drug Development in India’, highlights how non-animal methods, including human cell-based models, organ-on-chip technologies and computer simulations, can strengthen drug research by better predicting how medicines will work in humans.The report has been jointly developed by Humane World for Animals India, DBT-In-STEM, Animal Law and Policy Network and Dr Reddy’s Laboratories.
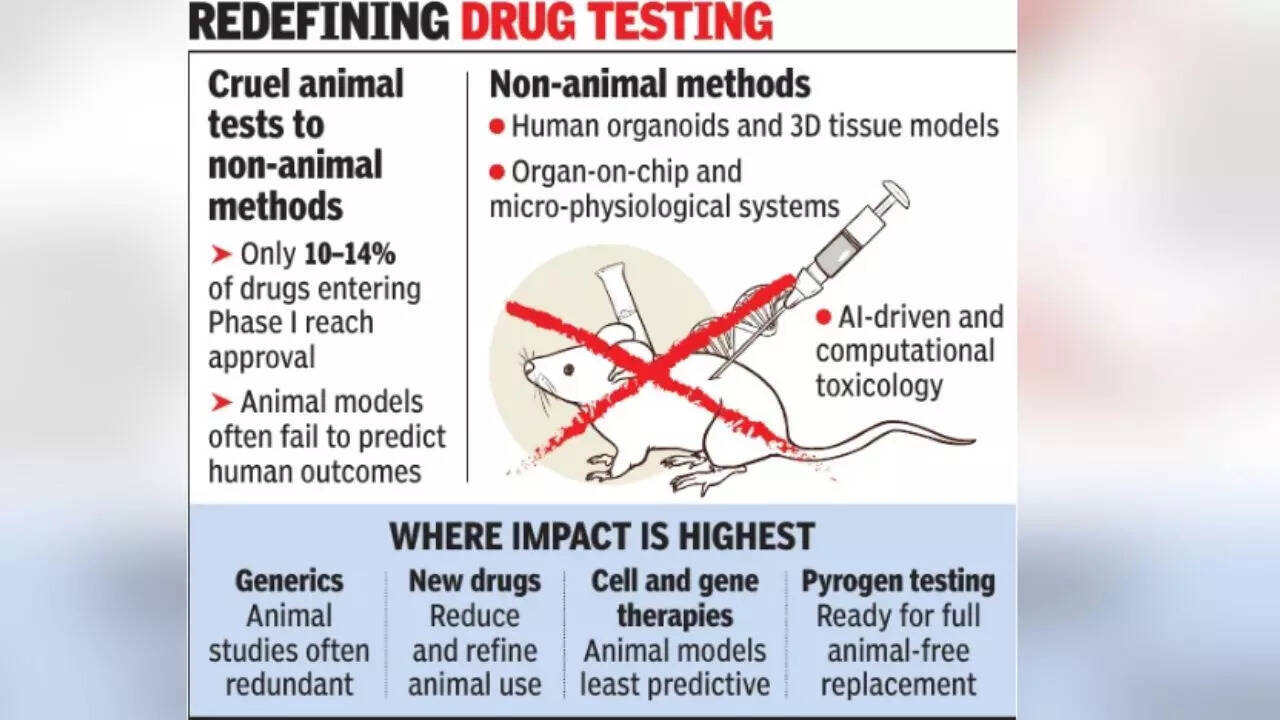
Scientists note while animal testing has traditionally been central to pre-clinical research, differences between animal and human biology mean that nearly 90% of drugs that enter early human trials fail, leading to higher costs, longer timelines and the loss of potentially useful therapies. The report stresses species-specific differences in physiology and genetics limit the ability of animal testing to predict the effect of drugs in humans.India’s regulatory framework has reached an “inflection point”, driven by re-forms such as the New Drugs and Clinical Trials (Amendment) Rules, 2023, which permit the use of human-biology-based alternatives like organoids, organ-on-chip systems and computational modelling, the report states.Its recommendations concern generic medicines, an area where India supplies about 20% of the global market.It notes that mandatory animal toxicity studies for generics often add little scientific value. “In contrast, regulators in the US and Europe routinely approve generics based on bioequivalence and existing safety data, without requiring fresh animal experiments. Aligning Indian rules with such global practices could reduce duplication, cut costs and speed up access to affordable medicines,” the report states.While the study does not favour a wholesale replacement of animal testing, with concerns relating to new chemical and biological entities, it instead proposes risk-based reduction and refinement.For cell and gene therapies, where animal models often poorly reflect human biology, the report recommends greater reliance on studies and methods to avoid repeat animal testing.It states that some non-animal methods, such as the monocyte activation test and recombinant factor c assay, can fully replace rabbitand horseshoe crab-based tests, which are already accepted in major pharmacopoeias worldwide.The report calls for a national strategy, stating that while India has strong capabilities in pharmaceuticals, biotechnology, and AI, there are major gaps, including the absence of a national validation framework, fragmented biobanking infrastructure and uncertainty over regulatory acceptance of non-animal data. It recommends setting up a central national agency for non-animal methods, dedicated funding, clear regulatory guidance and workforce training.“The time has come for conversations around alternatives to animal testing to move to action,” said Alokparna Sengupta, managing director, Humane World for Animals India, adding that human-relevant models can reduce drug failures, save animals and make development of medicines faster and more cost-effective.Dr Arvind Ramanathan, a scientist at DBT BRiC – Institute for Stem Cell Science and Regenerative Medicine (InStem), said non-animal testing can improve safety and efficiency while reducing reliance on animal models. Prof Sarfaraz K Niazi of University of Illinois pointed to a global shift among regulators towards science-led, human-relevant approaches.Unveiling the report through a virtual conference, Dr Reddy’s Laboratories chairman Satish Reddy said a transition towards humanrelevant testing methods could strengthen innovation while improving efficiency and predictability in drug discovery. Dr Reddy’s Laboratories CEO Deepak Sapra said adopting these methods can lower development costs and shorten timelines.











