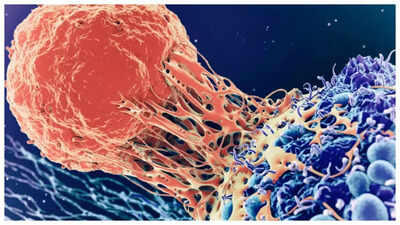
Russia has declared its readiness to use Enteromix as a personalised cancer vaccine for clinical applications, after successful development through mRNA technology similar to COVID-19 vaccines. The vaccine has demonstrated outstanding results in initial testing, because it uses advanced mRNA technology which powers COVID-19 vaccines. The treatment provides a safer method to fight deadly cancers, by training the body to identify and fight cancer cells. The Russian government will provide Enteromix at no cost to patients after receiving official approval, which represents a positive development for cancer treatment accessibility. Let’s find out more…What is EnteromixThe cancer vaccine Enteromix receives its unique characteristics from each patient’s RNA profile, which enables it to match the genetic features of their tumor cells. The vaccine developed by Russia’s National Medical Research Radiology Centre and Engelhardt Institute of Molecular Biology, uses mRNA technology to teach the immune system about cancer cell identification and destruction. The treatment method of Enteromix trains the body’s immune system to fight cancer, without producing dangerous side effects, which traditional chemotherapy and radiation therapy do.
Activate the immune responseThe vaccine contains four non-harmful viruses which activate immune response, while focusing on tumor destruction. The treatment strategy works to reduce tumor size and slow down cancer cell multiplication, while extending patient life expectancy. The initial version of the vaccine targets colorectal cancer, because it represents a major worldwide cause of cancer-related deaths. The development team has worked on multiple versions, which include glioblastoma brain cancer treatment, and specific melanoma therapies.Promising trial resultsThe vaccine underwent complete preclinical evaluation and initial clinical trials with 48 participants, who demonstrated positive outcomes. The head of Russia’s Federal Medical and Biological Agency (FMBA) Veronika Skvortsova reported that Enteromix achieved the following results during clinical testing:The treatment resulted in tumor size decreases ranging from 60% to 80% in patients.The treatment caused tumors to stop expanding and reduced their existing size.The treatment resulted in better survival rates among patients who received it.The vaccine proved to be safe, because it did not produce any dangerous adverse reactions during testing.The treatment proved effective during multiple administration sessions.The vaccine received public disclosure at the 10th Eastern Economic Forum in Vladivostok, during September 2025 when it drew interest from 8,400 delegates representing 75 countries. The FMBA needs official clearance to start using Enteromix for widespread medical treatment and distribution.A distinct mechanismThe standard cancer treatments of chemotherapy and radiation therapy work to kill cancer cells, but they also harm normal cells which results in major side effects including nausea, hair loss and weakened immunity. The immune system receives direct training through Enteromix to identify cancer cells as threats, which it can then eliminate. The immunotherapy model provides patients with reduced side effects and improved tolerance to treatment.The vaccine’s individualised design allows doctors to create doses that precisely match each patient’s tumor characteristics, which could result in better treatment results. The method provides a solution to the common problem of standard cancer therapies which fail to work for various cancer types.The worldwide development of Cancer vaccinesThe Russian Enteromix vaccine represents one of multiple worldwide initiatives, which use mRNA technology to create cancer vaccines. BioNTech and Moderna work on individualised cancer vaccines for pancreatic, lung cancer, and melanoma, which are currently in advanced stages of clinical development.The United Kingdom operates its Cancer Vaccine Launch Pad for large-scale vaccine trials, but India works on breast, oral and cervical cancer vaccines, while Russia stands out for its claim of clinical readiness. The approval of Enteromix would establish it as one of the first personalised mRNA cancer vaccines, which could reach public markets.What this means for patientsEnteromix has the potential to transform cancer treatment for millions of colorectal cancer patients (and others), in the upcoming years if it gains regulatory approval. The treatment shows potential to enhance both survival rates and patient life quality, because it delivers targeted therapy with minimal adverse reactions. The Russian government plans to distribute Enteromix without charge to patients because they want to enhance advanced cancer treatment availability for all social classes.Challenges and next stepsEnteromix requires additional phase 2 and phase 3 clinical trials with various patient groups, to validate its safety and effectiveness for widespread use. The vaccine needs to pass additional safety and effectiveness tests through phase 2 and phase 3 clinical trials, before it can be used by the general public. The Russian Ministry of Health and worldwide health organizations need to approve Enteromix for use as a vaccine.